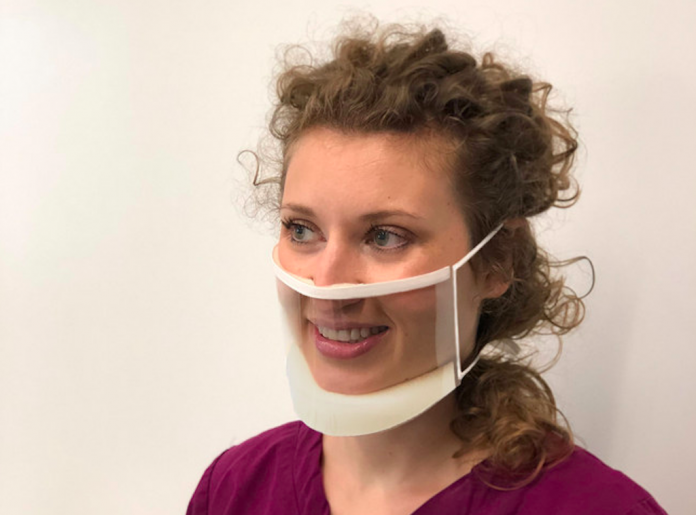
American medical supply company Clearmask LLC has announced that it received the U.S. Food and Drug Administration’s 510(k) clearance for its ClearMask™, a fully transparent surgical mask that eases communication barriers in healthcare settings.
ClearMask™ is the world’s first FDA-cleared, fully transparent surgical mask that can be used in hospitals, clinics, schools, retail, hospitality, and other settings. The mask is optimised for maximum clarity and comfort, and meets applicable ASTM Level 3 requirements for fluid resistance and flammability, which offers a high level of protection for medical use in environments such as operating rooms.
Allysa Dittmar, President of ClearMask, said the company is now ready to join the fight against the current pandemic.
“After three years of research, development, and testing, we are thrilled to bring a human-centered mask to everyone who needs it, especially those who can benefit from improved visual communication, such as children, older adults, deaf and hard of hearing people, and those who do not speak the same language. The ClearMask™ is well-positioned to join the fight against the current pandemic,” said Ms Dittmar.
The company, consisting of Johns Hopkins University graduate students and alumni, started developing the transparent mask in 2017 after their deaf co-founder experienced an adverse experience during her surgery. Traditional surgical masks blocked her providers’ faces, impeding effective communication and safety.
“ClearMask™ helps provide protection while bringing much-needed relief through a reassuring smile and familiar face among fear, confusion, and suffering,” the company said in its press release.
“Transparent communication during the customer experience can be critical in establishing rapport and earning trust, while assuring safety as a priority.”
Meanwhile Aaron Hsu, CEO of ClearMask said: “Regulatory clearance and mass production are two significant milestones in ClearMask’s mission to get the ClearMask out to as many people as possible. This achievement is a testament to our company’s hard work and commitment to serving different communities in need during this time.”
The ClearMask™ is the first product in ClearMask LLC’s growing portfolio of novel masks to receive FDA clearance as a Class II medical device. So far the company has provided the masks in bulk volumes, typically in the tens to hundreds of thousands. The consumer masks can now be purchased through ClearMask’s website at buy.theclearmask.com, starting at a box of 24 masks.




















