Image credit: www.qut.edu.au
A team of biomedical scientists from The University of Texas and The QUT have discovered the key physiological process that prevents the successful integration of 3D printed replacement body parts for many patients.
Image credit: www.qut.edu.au
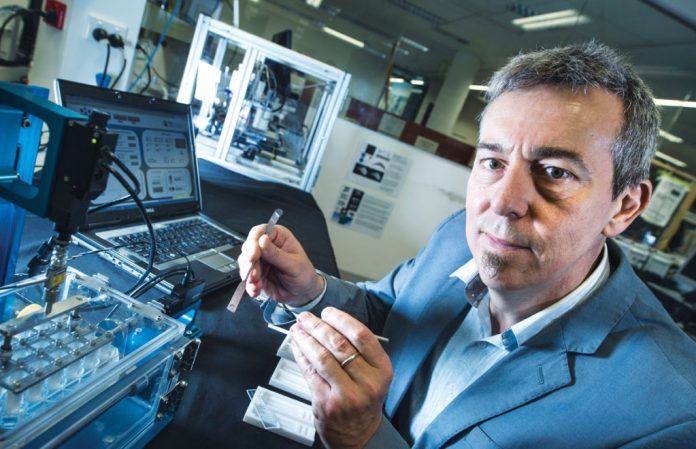
Professor Dietmar W Hutmacher, from QUT’s Australian Research Centre in Additive Biomanufacturing said the patient’s body often rejects medical devices and implants as it perceives them as foreign objects.
“These implants elicit a foreign body response (FBR) and cause fibrotic encapsulation or scar tissue to develop around the implant which impairs its intended function in the body,” Mr Hutmacher explained.
He said the team had to better understand the mechanisms behind the body’s response before finding ways to intervene so the 3D printed biodegradable scaffold could slowly dissolve away as it is replaced by new living tissue at later stages in the regeneration process.
“We know that when tissue is exposed to biomaterial it triggers a step-wise process of inflammation, followed by wound healing and, if not resolved from a pro-inflammatory to a pro-reparative or regenerative mode of action, tissue fibrosis and scarring,” Professor Hutmacher continued.
He said the team studied the mechanistic roles of two white blood cell types – monocyctes and macrophages – key to the body’s regeneration process.
“There are two types of macrophages – M1 macrophages cause inflammation and M2 macrophages are pro-regenerative. Both are implicated in the formation of fibrous scarring around the implant yet we did not fully understand the mechanism by which they either enhanced or counteracted the formation of fibrotic scarring in the context of an additive biomanufactured scaffold,” the Professor added.
“Our research into their role found that M1 macrophages joined and became immobilised along the scaffold surface before forming multi-nucleated giant cells which initiated a network of neovessels within the scaffold pores that acted in a way similar to the neovessels that support the growth of fibrous tissue around a tumour. These macrophages and their giant cells produced a growth factor which led to the formation of the dense collagen capsule around the implant two to four weeks later.”
He said these insights into the scaffold integration process within soft tissue suggested therapeutic intervention.
“We found that administration of clodronate, an anti-osteoporosis drug, and VEGF Trap, an anti-vascular endothelial growth factor drug, significantly reduced giant-cell accumulation, and the formation of neovessels and fibrosis,” Professor Hutmacher continued.
“This represents a promising strategy to modulate both early inflammation and later stage tissue remodelling (weeks to month) to minimize chronic FBR and implant failure.”




















