MIT scientists have developed a new method for converting carbon dioxide into useful compounds such as fuels and feedstocks for a variety of manufacturing industries.
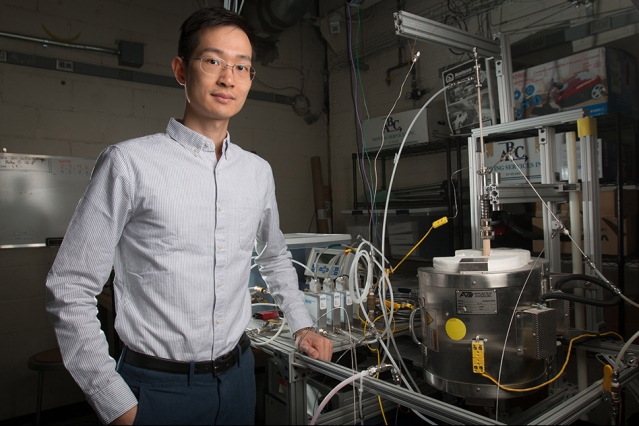
Developed by MIT postdoc Xiao-Yu Wu and Ahmed Ghoniem, the Ronald C. Crane Professor of Mechanical Engineering, the new membrane-based system turns power plant emissions of carbon dioxide into fuels for cars, trucks, and planes, as well as into chemical feedstocks for a wide range of products.
The system features a membrane – which is made of a compound of lanthanum, calcium, and iron oxide – that allows oxygen from a stream of carbon dioxide to migrate through to the other side, leaving carbon monoxide behind.
“The membrane, with a structure known as perovskite, is 100 percent selective for oxygen, allowing only those atoms to pass,” reads MIT’s media statement.
“The separation is driven by temperatures of up to 990 degrees Celsius, and the key to making the process work is to keep the oxygen that separates from carbon dioxide flowing through the membrane until it reaches the other side. This could be done by creating a vacuum on side of the membrane opposite the carbon dioxide stream, but that would require a lot of energy to maintain.”
According to MIT researches, the carbon monoxide produced during this process can be used as a fuel by itself or combined with hydrogen and/or water to make many other liquid hydrocarbon fuels as well as chemicals including methanol (used as an automotive fuel), syngas, and so on.
Mr Wu said the entire process is driven by heat, which could be provided by solar energy or by waste heat.
“Essentially, the process makes it possible to store that heat in chemical form, for use whenever it’s needed,” he explained.
“Chemical energy storage has very high energy density — the amount of energy stored for a given weight of material — as compared to many other storage forms.”
He said the process can work with any level of carbon dioxide concentration, adding that ‘the higher the concentration, the more efficient the process is’.
“So, it is well-suited to the concentrated output stream from conventional fossil-fuel-burning power plants or those designed for carbon capture such as oxy-combustion plants,” he concluded.
The research, which is described in a paper in the journal ChemSusChem, was funded by Shell Oil and the King Abdullah University of Science and Technology.