Australian medical device innovator Epiminder has raised $16 million to expand its ongoing UMPIRE (sUb-scalp Monitoring ePileptic seIzuREs) clinical trial for Minder®, an ultra-long-term ambulatory electroencephalography (EEG) monitoring device.
According to the official announcement, in addition to expanding its UMPIRE clinical trial to multiple clinical sites in Victoria, Queensland and New South Wales, the company will also expand product development, manufacturing and corporate activities.
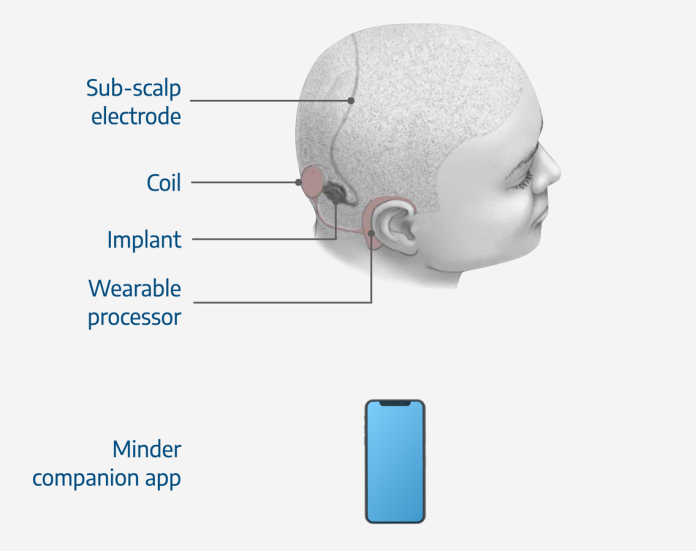
Minder® is a minimally-invasive device for long-term monitoring of brain seizures which provides detailed data on seizure activity and frequency to both patients and their doctors. The device can be worn by patients throughout the day and does not interfere with regular daily activities.
“We are extremely pleased by the results obtained to date demonstrating significant long-term cycles of brain activity. These cycles are allowing Epiminder and its partner Seer Medical to forecast seizure risk. Initial seizure forecasting results have recently been published in Frontiers of Neuroscience,” said Professor Mark Cook, St Vincent’s Hospital neurologist and chair of medicine at the University of Melbourne.
“With the bridge round financing completed and the expansion of the UMPIRE clinical trial underway, Epiminder is well positioned to advance the Minder® ultra-long term epilepsy monitoring device. With our strategic partners from around Australia, Epiminder aims to revolutionise epilepsy care for millions of people around the world,” said Rohan Hoare, Chief Executive Officer of Epiminder.
The company’s shareholder is medical device leader Cochlear.
Back in 2020, the company raised $AU18 million from private investors as well as the current shareholders: Cochlear, the Bionics Institute, Melbourne’s St Vincent’s Hospital and the University of Melbourne.



















