Researchers from the University of Sydney have shown that oil molecules retain their ‘liquid-like’ features when they are chemically bonded as an incredibly thin layer to solid surfaces, creating new opportunities for developing sustainable materials with non-stick characteristics.
The findings are published in the chemistry journal Angewandte Chemie, led by Dr Isaac Gresham, with co-authors Professor Chiara Neto and honours student Seamus Lilley from the School of Chemistry and Sydney Nano, Dr Kaloian Koynov from the Max Planck Institute for Polymer Research and Dr Andrew Nelson from the Australian Centre for Neutron Scattering.
‘Liquid-like’ coatings, also known as slippery covalently-attached liquid surfaces (SCALS), are made of silicones or polythene glycol, both of which degrade into harmless byproducts in the environment, according to the study.
SCALS are anti-adhesive without the usage of hazardous perfluorinated polymers (PFAS), sometimes known as ‘forever chemicals’ and commonly employed for their low adhesion capabilities.
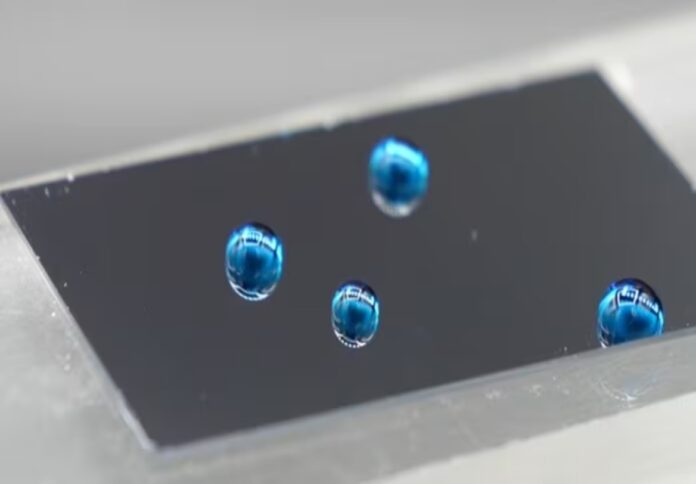
“These liquid-like layers are extremely slippery to most contaminants: they shed liquid droplets effortlessly, which is great to increase the efficiency of heat transfer and for collecting water, they prevent the buildup of scale, and resist the adhesion of ice and bacteria, bringing us one step closer to a self-cleaning world,” said Professor Neto, who leads the Nano-Interfaces Laboratory at the University of Sydney.
Prof Neto added that the outstanding performance of these layers may be correlated with their nanostructure, which means researchers now know what they are striving for when designing slippery surfaces, allowing them to even be more effective and give viable alternatives to fluorinated coatings.
Researchers said the slippery nano-thin layers are made up of oil molecules that are only a hundred atoms long and are between two and five billionths of a metre thick, or 10,000 times thinner than a human hair.
“A water droplet glides with no friction over a thick oil film, but if you completely remove the oil film, say by using soap, most water droplets will stick to solid surfaces,” Professor Neto said.
In particular, the researchers employed two approaches to ‘see’ the surface layers of their ultra-thin liquid coatings to unlock their secrets.
The first method is single-molecule force spectroscopy, which determines the length of individual molecules as well as the force needed to stretch or compress them.
The second technique is neutron reflectometry, which lets scientists determine the length and density of grafting in molecules.
“We found that if the liquid molecules were too short and sparsely grafted on the solid surface, they did not adequately cover the underlying solid surface and remained sticky,” Prof Neto said.
She added, “On the other hand, if molecules were too long or grafted too densely, they did not have enough flexibility to act like a liquid.”
“For SCALS to be effective, they needed to be in a Goldilocks zone, where they are neither too short nor too long, nor packed too loose or too tight.”
The scientists also examined the rate at which a tiny probe molecule diffused into the layer in order to demonstrate conclusively that the extraordinary features of these layers are caused by their ‘liquid-like’ condition.
Professor Neto concluded that the quickest molecular diffusion was recorded in the Goldilocks zone, where the oil molecules are just the appropriate length and densely grafted.
















