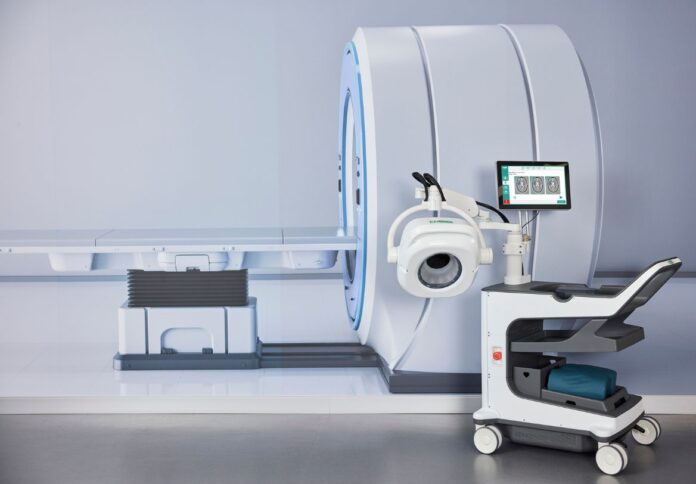
Brain imaging company EMVision Medical Devices Limited has received a cash progress payment of $1,750,000 under its Modern Manufacturing Initiative (MMI) grant award.
The ASX-listed company revealed that it had received $5 million in non-dilutive funding from the Federal Government’s MMI Medical Products Translation stream to begin commercial manufacture of EMVision’s world-first portable brain scanner 1st Gen device.
“Pleasing progress has been made against project milestones under the program, including design verification, progression of systems testing and certification, and preparation of technical documentation for release of the product in its multi-site clinical trials,” EmVision said in an ASX announcement.
According to the company, efforts to set up production layout and processes, together with work and test instructions, had also made good progress and served as prerequisites for actions to verify and validate products as part of regulatory filings.
These are requirements for the establishment of commercial manufacturing capabilities as well as for the product verification and validation processes that are a part of regulatory submissions, EmVision noted.
“The final project milestones ‘manufacturing capability established’ and ‘first production run’ are anticipated to be achieved before May 2024 under the program, which is when the final grant payment of $1,250,000 is due,” the ASX-listed company said in a statement.
Last month, EMVision moved forward to stage two of the multi-site trial for its first-generation portable brain scanner.
The Brisbane-based company announced stage two would see it enrol 150 acute stroke and stroke-mimic patients across major stroke centres in Australia, including Liverpool Hospital in New South Wales, Royal Melbourne, and Princess Alexandra in Queensland.
The announcement comes following the completion of stage one which included 30 healthy volunteers.