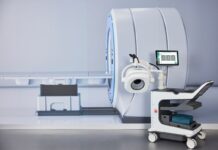
Australian medical device company EMVision Medical Devices Limited announced the successful assembly of an advanced 28-antenna prototype for its 2nd Gen ultra-lightweight helmet scanner.
The ASX-listed company said the new product is tailored for deployment in road and air ambulances, representing a major leap forward in first responder brain imaging capabilities.
EMVision aims to address a significant clinical need with its complementary product suite, which caters to bedside (1st Gen) and first responder (2nd Gen) brain imaging.
The 2nd Gen helmet scanner boasts cutting-edge features, including a weight of under 10 kilograms, making it ideal for transportation to the point of care via a backpack.
It is equipped with a 28-antenna 3D array designed to offer complete brain coverage in a single scan, supported by high-performance ultra-lightweight antennas.
The device incorporates a silicone membrane with coupling media to ensure efficient antenna-head connectivity.
The membrane is designed to be reusable but replaceable, while the coupling media will be a consumable item per scan.
To maximize efficiency, EMVision plans to adapt and update the core algorithms developed for the 1st Gen device for use in the 2nd Gen model.
To ensure the effectiveness and practicality of the new technology, EMVision is embarking on a rigorous bench testing phase.
This testing will evaluate a range of technical parameters and compare the results to simulations, ultimately leading to target detection investigations.
Additionally, an ethics submission for healthy human volunteer testing has been made, with testing expected to commence in the coming months.
This phase will assess usability, ergonomics, and signal benchmarking, providing valuable insights into the helmet scanner’s real-world performance.
From a regulatory perspective, EMVision’s strategy for the 2nd Gen device is to leverage the 1st Gen device as a predicate.
This approach will enable the pursuit of FDA 510(k) clearance, a pathway that demands demonstrating “substantial equivalence” to a previously approved device in terms of safety and effectiveness.
This strategy streamlines the regulatory process and ensures a smoother path to market for EMVision’s innovative technology.
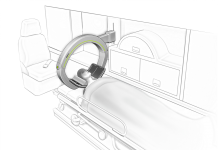
According to EMVision, this highly advanced prototype serves as a forerunner to the ‘proof of concept’ system, as illustrated below, designed for deployment before reaching the hospital.
It is progressing as scheduled to be constructed during the initial half of the calendar year 2024, aligning with the company’s development schedule.
Subsequently, it will be employed for the scheduled trials in road and air ambulances as part of EMVision’s partnership with the Australian Stroke Alliance.